Just like all GMP advice facts, it is often precious to test to know the fundamental principles to respond in a method that each fulfills the GMP requirement or expectation and strengthens the quality system with greatest profit on the affected person.
Nelson’s claim that large marketing implies exceptional quality is, consequently, not supported with the accessible evidence. In actual fact, in the the latest survey of shopper attitudes the vast majority of respondents felt that marketed products ended up no far more prone to be dependable than had been products with no promotion. fifty
Investments in machinery and equipment need to bring about much more regular production in addition to enhancements in employee productivity. Quality improvements are also predicted to cause more price savings, in the shape of working experience-primarily based scale economies, by way of their influence on sector share and (cumulative) production levels.59
The review prerequisites in the EU PQR for MA variants, currency of complex agreements, as well as the postmarketing commitments don't replicate The standard field practice for PAR/PQR, and there have been field remarks that Many of these review prerequisites appeared to be exterior the scope of the PQR. The review requirements for MA and postmarketing commitments mirror the extensive-standing EU emphasis on license compliance and the heightened global emphasis on drug security, respectively. The MA or, specifically, the marketing and advertising authorization software (MAA) may be the product license while in the EU comparable to the new drug application (NDA) during the US. In the course of an inspection, it can be usual for an EU inspector to issue the business's management regarding their expertise and assurance of commitments created from the MA.
This approach lends a vertical or hierarchical dimension to quality, for merchandise could be rated in accordance with the amount of the desired attribute they have. However, an unambiguous ranking is feasible only if the attributes in concern are regarded as preferable by pretty much’ all purchasers. 7
Welcome to EEC's Qualified Skills Registry (PQ Registry). Educators working in packages serving youngsters from beginning by school age, regardless of location, can generate someone educator profile inside the PQ Registry. EEC encourages all educators to make the most of the PQ Registry, including individuals who operate in public preschools as well as other systems that are not subject matter to EEC licensure. Should you be currently Doing the job in early schooling or out-of-faculty time in an EEC-accredited Centre-based method or loved ones child treatment dwelling in Massachusetts, you have got to sign-up to comply with the 2010 Family, Team and faculty Age Little one Treatment Laws. Educators, together with assistants, who perform with infants, toddlers, preschoolers, or school age kids in EEC-accredited settings are necessary to register and update their registration annually. EEC is additionally demanding systems that are not matter to EEC licensure to sign up their educators whenever they wish to participate in EEC's Quality Score Advancement Process (QRIS). EEC's new Expert Skills Registry gathers essential info on the scale, composition, education, and working experience of our recent workforce. It suppliers information about the retention and turnover of educators working in early education and learning and out-of-faculty time plans. This information and facts will help EEC develop a workforce growth process that responds on the wants of all educators and systems in Massachusetts.
If the amount of batches is fewer (fewer than 3) within the review time period Product Quality Review (APQR) could be compiled for 2 years with the two yr facts with proper justification, if demanded.
Name of your suppliers/companies of your elements, address element of broker/distributor/agent & Review the element like modify in route and manner of transportation and transit problem.
Cross-reference: Warning Letters mentioning deviations from balance tests suggestions, insufficient data integrity, or failure to update shelf daily life based on new data.
Both dependability and conformance are carefully tied into the producing-centered approach to quality. Advancements in both steps are Generally considered as translating instantly into quality gains due to the fact defects and area failures are regarded as undesirable by virtually all people.
Enhanced Affected individual Security: By protecting stringent quality requirements and addressing possible hazards, APQR contributes to the security of sufferers who depend upon pharmaceutical products. This underscores the dedication to client properly-staying.
This instance indicates the necessity of carefully concentrating on just one’s quality area of interest. The choice of a defensible area of interest, nevertheless, is barely a initial step. Operational requirements ought to even be met, for each dimension of quality imposes its read more own requires click here within the organization. Higher performance needs thorough focus to style and a robust structure workers; remarkable longevity needs the usage of prolonged-lived or “derated” elements and close cooperation among the engineering and getting departments; top-quality conformance demands awareness to written specifications and precision in assembly; and Excellent serviceability requires a sturdy customer care Office and Energetic discipline representatives.
Check no matter if investigation is documented in the event of batches not Conference the produce Restrict and check if the root cause has become determined and whether or not corrective / preventive motion/s taken had been ample.
Japanese manufacturers, on the other hand, have succeeded in generating products that fulfill the dual goals of substantial quality (conformance and dependability) and cheap. Their capacity to achieve this has compelled analysts to rethink the concept of manufacturing tradeoffs, For several standard assumptions now not use.seventy five This space Obviously warrants additional exploration. Tradeoffs amongst the various Proportions of quality and concerning these dimensions along with the targets of Price, adaptability, and shipping and delivery must be far better understood. Do the various dimensions of quality have to have various sorts of expertise, or are companies likely to succeed on many Proportions without delay?
 Danny Tamberelli Then & Now!
Danny Tamberelli Then & Now! Michael C. Maronna Then & Now!
Michael C. Maronna Then & Now! Kelly Le Brock Then & Now!
Kelly Le Brock Then & Now!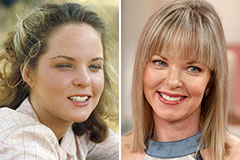 Melissa Sue Anderson Then & Now!
Melissa Sue Anderson Then & Now! Ricky Schroder Then & Now!
Ricky Schroder Then & Now!